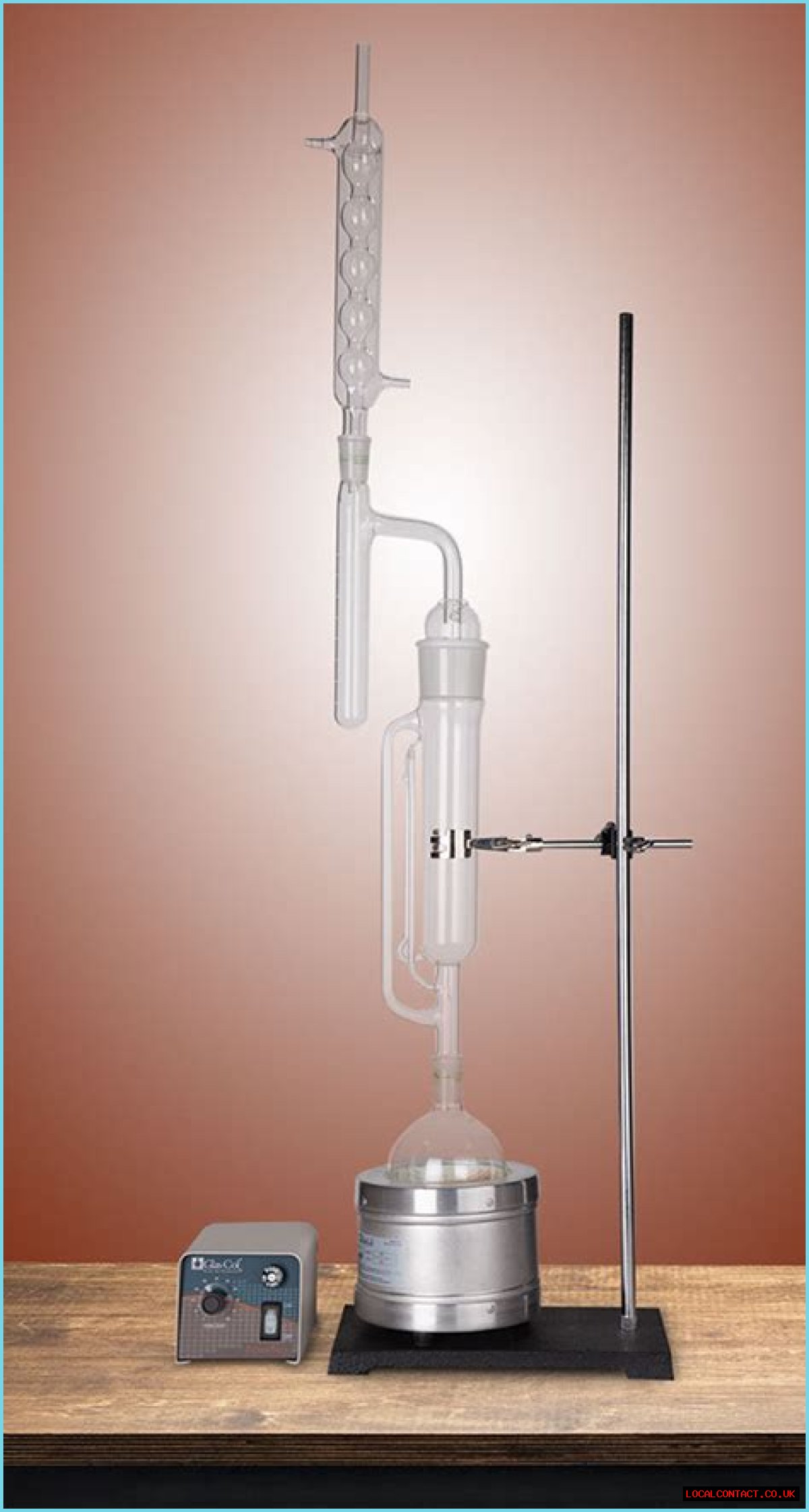
What is Dean-Stark apparatus used for?
The Dean–Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water (or occasionally other liquid) from a reactor.
How does Dean and Stark method work?
A method for the measurement of fluid saturations in a core sample by distillation extraction. The water in the sample is vaporized by boiling solvent, then condensed and collected in a calibrated trap. This gives the volume of water in the sample.
Why is one arm of the Dean-Stark usually insulated with aluminum foil?
As with standard Dean–Stark technique, it is best to insulate the reac- tion vessel and addition funnel side arm with cotton and alu- minum foil so as to maximize reflux efficiency.
👉 For more insights, check out this resource.
What is distillation apparatus?
The distillation apparatus, commonly called a ‘still’, consists of a vessel for plant material and water, a condenser to cool and condense the vapour produced and a method of collection, or ‘receiver’. This is then heated to boiling point and the steam (water vapour) carries out the volatile oils.
👉 Discover more in this in-depth guide.
How do you remove water from toluene?
Toluene can be predried using CaCl2, CaH2, or CaSO4 and is most commonly dried by heating over sodium with benzo- phenone as indicator. 1 Such treatment (Na/benzophenone) reduced the water content from 225 ppm to about 34 ppm in our hands (Table 2).
Why is toluene used in Dean Stark apparatus?
This is an advantage when using the Dean-Stark trap to influence equilibria, because it will ensure the continuous removal of water. Upon heating the reaction mixture, the formed toluene/water azeotrope will distill over, condense in the condenser, and flow into the Dean-Stark trap.
What equipment do you need for distillation?
Thre are three essential pieces of distillation equipment needed to carry out the process; Reboiler or Pot – Used to heat the source liquid. Condenser – The heated vapour is cooled back to a liquid state. Receiver Flask – The device into which the concentrate/distillate is collected.
What equipment is used in simple distillation?
A simple distillation apparatus consists of a boiling flask (round-bottom flask) attached to an adapter holding a thermometer (to determine the boiling temperature of the liquid). The adapter connects to a condenser into which cold water is constantly passed through.
How is residual toluene removed?
running the medium through activated charcoal should remove the toluene. you might need to use fairly high quality charcoal. Alternatively if you only want to extract the toluene for anlaysis then you can re-extract the toluene from the charcoal afterwards.
How do drying agents remove water?
For the most common drying agents such as sodium sulfate or magnesium sulfate, the crystals form larger clumps when they absorb water. After standing for a short period the crystals are removed by filtration or decantation, and the solution is then relatively free of water.
What is toluene distillation method?
Moisture is removed from the sample by distillation as an azeotrope with toluene. The water is collected in a suitable trap and its volume is measured at a known temperature. SCOPE. This method is applicable to all unmodified starches, most modified starches and many starch products (Note 1).
How does a Dean Stark distillate work?
This innovative and compact Dean-Stark receiver is designed for azeotropic distillations using solvents that are heavier than water. The solvent vapor travels through tube “A” to a condenser inserted in the top standard taper outer joint.
What is the purpose of the Dean Stark/Soxhlet extracion apparatus?
Environmental- Use this Dean Stark/Soxhlet extracion apparatus to do Soxhlet Extractions and Dean-Stark Azeotropic Distillation in a single process. Determines of both both water and dioxins on the same sample aliquot is achieved without the need for desiccation prior to Soxhlet extraction.
What is modified Dean-Stark trap?
Modified Dean-Stark trap allows for more efficient cooling and separation of the azeotrope during distillation. Graduated from 0 to 10ml in 0.2mL subdivisions. **Design suggested by Dr. Craig Merlic, University of California, Los Angeles, California
How do you distill azeotrope with Dean Stark trap?
Simply add a condenser to top outer joint, any graduated funnel from 125mL to 2000mL to bottom inner joint, attach sample flask to inner side arm joint and you have a moisture test receiver. Modified Dean-Stark trap allows for more efficient cooling and separation of the azeotrope during distillation.